-
Full-process coverage
One-stop solution:Wet experiments, digital slide reading
Full-platform coverage:HE, IHC, FISH, multiplex immunofluorescence
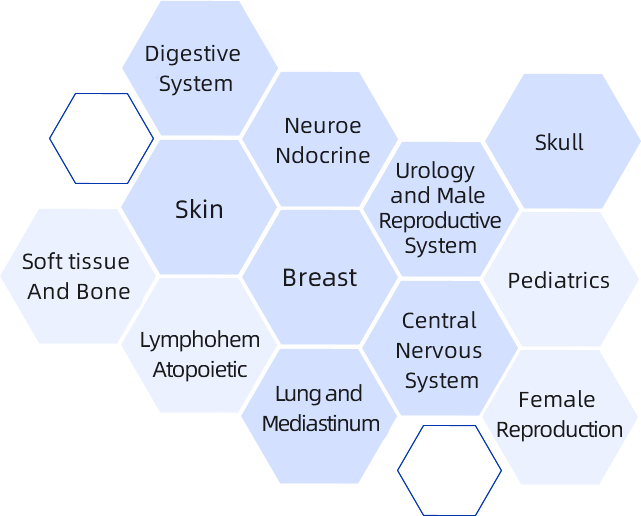
Full-disease coverage:12+ pathology sub-specialties for common and rare disease diagnosis.
Full-scenario support:PCR (pathology CR), MRP....
-
Clinical decision-driven
Evidence chain: From biomarker discovery to patient stratification and efficacy validation.
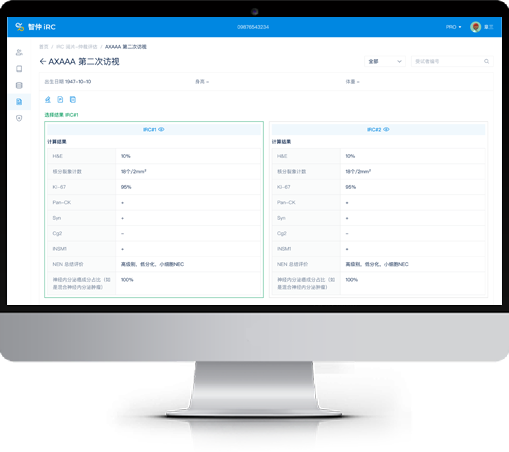
Strict quality control:Objective and traceable results via IRC review and 4-level quality system.
Global compliance:Meets FDA/NMPA standards, supports China/US/EU trials
Efficient outcomes:High-quality data for registration and publication
-
Competitive advantage
Top Expert Network:100+pathologists
Global Quality Accreditation:CAP/CNAS-certified laboratories.
Agile Service System: Urgent-case prioritization to meet your timelines
Intelligent Data Platform:Meet global standards and complete data tracking